They can also act as the nucleophiles for the reaction.
Do vinyl go through sn2 reactions.
In a back side.
Sn1 is a two stage system while sn2 is a one stage process.
The student asked why do vinyl halides not do the sn2 reaction my answer was that two reasons exist for why the vinyl halide will not react with a nucleophile.
The reaction most often occurs at an aliphatic sp 3 carbon center with an electronegative stable leaving group attached to it often denoted x which is frequently a halide atom.
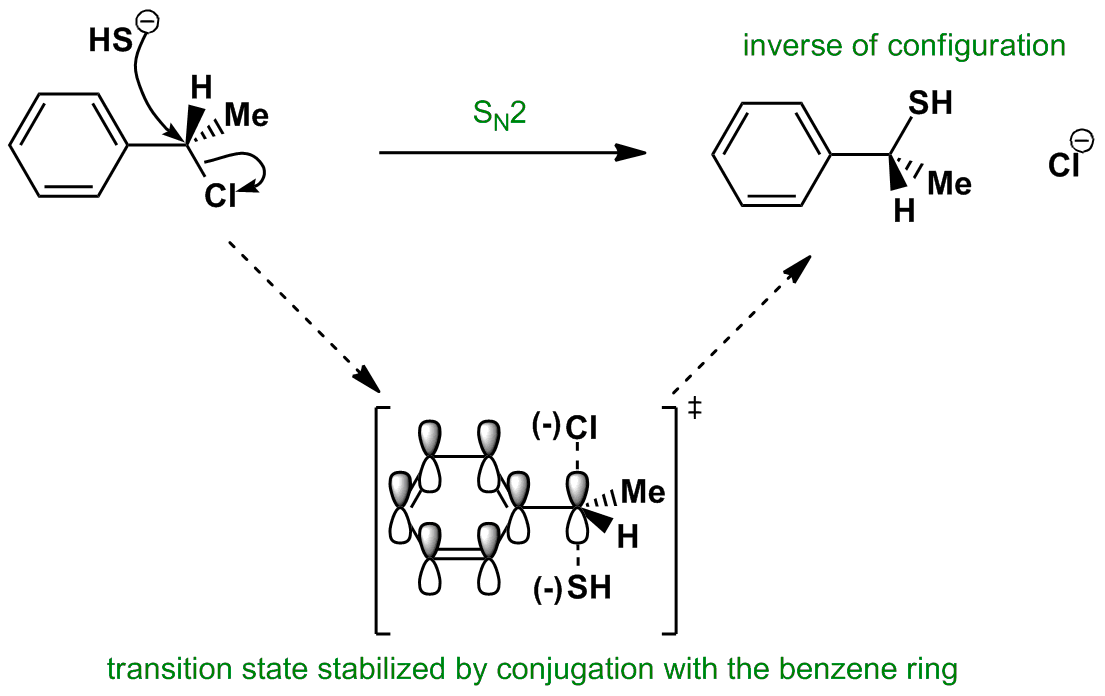
After the leaving group leaves the other substituents shift to make room for the newly bonded nucleophile changing the stereochemistry of the molecule.
The s n 2 reaction notes.
Solvolysis of vinyl halides in very acidic media is an example.
This is called a back side attack.
It covers the way the reactants are joined up together through transition states and how they transform into the reaction products.
A chemical species that donates an electron pair to an electrophile to form a chemical bond in relation to a reaction.
Today i got a good question i want to make a point of posting the best question from the day s teaching and my answer.
The breaking of the c x bond and the formation of the new bond often denoted c y or c nu occur simultaneously through a transition state in which a carbon under nucleophilic attack is.
Not a nucleophilc substitution reaction the nature of the nucleophile in the sn2 reaction.
R1 x nu x nu x nu x r2r1 r3 ch3 2culi ch3 r2r1 r3 from chapter 10.
What determines sn1 or sn2.
The picture below helps explain why this reaction is so much more difficult energetically more costly than the more common solvolysis of an alkyl halide.
The carbocation can form as an intermediate during sn1 reactions while it is not formed during sn2 reactions.
A consequence of the concerted bimolecular nature of the s n 2 reaction is that the nucleophile must attack from the side of the molecule opposite to the leaving group.
Sn2 reaction showing concerted bimolecular participation of nucleophile and leaving group.
In the rate of reaction sn1 reactions are unimolecular and have a step wise mechanism.
Behind the leaving group.
The measure of nucleophilicity is imprecise.
A simple substitution reaction can go through two basic types of sequences or reaction mechanisms.
In organic chemistry a reaction mechanism is the step by step sequence in which a reaction takes place.
The resultant vinylic carbocations are actually stable enough to be observed using nmr spectroscopy.
This back side attack causes an inversion study the previous slide.
Increasing reactivity in the sn2 reaction vinyl and aryl halides do not react in nucleophilc substitution reactions x r2r1 r 3 nu.